
OK, here’s my original idea about how to turn salt water or polluted water into fresh water. You may say, “Perkel, you’re all wet.” But tell me why this wouldn’t work.
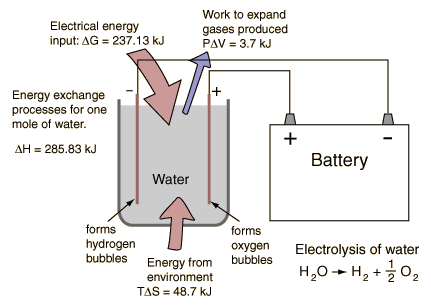
As you know – if we run electricity (DC) through water we can separate it into hydrogen and oxygen. We then take the hydrogen and oxygen and run that into a fuel cell which generates electricity and produces fresh water as a byproduct. And the water produced is FRESH WATER, and the electricity generated can be piped back to be used in the separation process.
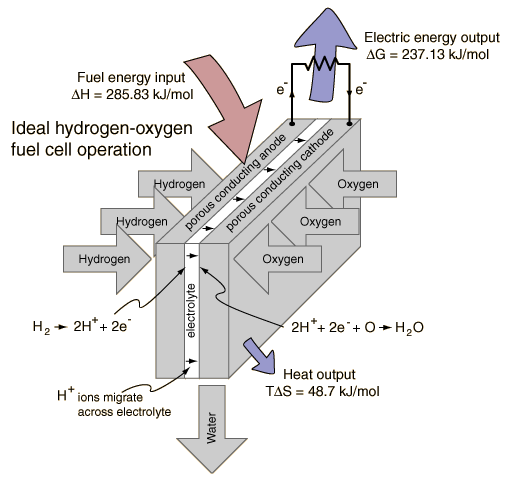
I’m not proposing a perpetual motion machine. I understand that the power produced by the fuel cell will be less than the power required to split the water in the first place. But this isn’t about producing power, it’s about producing clean water. The recycled electricity just makes it perhaps affordable. Salt water goes in, clean water comes out and the amount of electricity consumed would be maybe 1/5 (80% efficient?) of what it would be if you used electricity to boil the water and then condense the steam.
So – first question – why won’t this work? What am I missing?
Second question – efficiency? If electricity is used to separate water into hydrogen and oxygen and then converted back to electricity in a fuel cell, how much is lost? Anyone have any numbers?
How many gallons of water would you get per kilowatt hour of electricity? Makes you wonder if this were powered by some kind of wave or tidal generator to make the electricity to glean the water – how much water would you get out of this thing?














Mite work but i have a better idea.
Use a big stick and beat somebody until they ride a bike on a generator fast enough and generate enough power to desalinate the water.
Extra encouragement would be that they are only allowed to drink the water that comes out, so if they don’t push it hard enough they get to drink salty water only.
Prisoners could be used.. or politicians.
I have a feeling they would drink more water than they produced.
Just off the top of my head…
1. The electrolysis process is a chemical change; breaking the bonds between atoms in a molecule. This is bound to require substantial more energy than a distillation process which is only a state change (liquid to gas).
2. I suspect that the breaking and then recombination of water molecules process is a very low efficiency operation. I wouldn’t be surprised if we are looking at the 25% range. So while maybe some energy return is possible, not enough to compensate for the additional energy required for electrolysis over distillation.
3. I don’t know if electrolysis of salt water is clean. Won’t you get reactions like:
2 NaCl + 2 H2O → Cl2 + H2 + 2 NaOH
happening? What happens with the Chlorine and Lye?
Maybe there is a reason this hasn’t come about.
The problem is the salt, other electrolytic compounds, and trace disolved minerals. One of the primary problems with desalination is dealing with the scale left behind. Ever swim in the ocean, then dry out in the sun? All that cruchy (and itchy) stuff left behind on your swim trunks is what would eventually (probably fairly quickley) coat your electrodes and the sides of you container.
In addition, electrolysis performs most effeciently with a narrow range of electrolytic concentrations, so keeping the solution “just right” may be an issue.
Lastly, the gasses bubbeling up will most probably not be pure oxygen and hydrogen. If salt water is used, chlorine gas and sodium hydroxide are produced as well, which may destroy the fuel cell.
There are probably engineering soulutions to these problems, but you asked why this wouldn’t work :>
My understanding of this is
energy_1 + water_1 = Hydrogen + Oxygen
Hydrogen + Oxygen = energy_2 + water_2
the cost of energy_1 is assumed to be greater than the value of energy_2. This being the case, does the value of water_2 make up the difference with some left over for profit?
You will also get by-products from the initial creation of hydrogen and oxygen which will have costs and values associated with them.
If you can sell the water for more than it costs you to produce it then it will work
#3, 4 and 5, as I understand it, are correct.
Running electricity through salt water would produce a witches brew of noxious gases.
But how about this?
I mentioned before an idea which was floated around a few years back about placing military grade nuclear reactors off the shoreline of the US at regular intervals and using those reactors to generate electricity, hydrogen, and fresh water.
The only thing that nuclear reactors are good for is providing heat, and they are very good at it. They provide enough heat to drive large scale desalination, which pure water is used for drinking and in the boilers to drive the turbines, which turbines provide the electricity to crack some of the water into hydrogen and oxygen, which could be transported wherever needed.
This idea could easily eliminate most if not all coal fired powerplants while providing enough electricity to make electric cars a feasible reality.
Imagine, no more coal fired power plants spewing toxins and far, far less gasoline use. Not to mention complete energy independence!
Of course, the Environmental Lobby killed it. No joke.
If you electrolyze salt water you get chlorine gas and sodium, because the redox potential of that reaction is lower than splitting water into hydrogen and oxygen.
Please don’t try your experiment in a closed room. Chlorine gas can cause lung bleeding.
What about collecting condensation on a household level. All you need is a sheet of (recycled) plastic and a collection vessel.
PS
As a young lad, I was told that nuclear power would/could be the great savior.
Then I found out they were just blowing steam up my ass.
Hydrogen and oxygen are extremely intert molecules. If they combine, they combine in an explosion more powerful than in ICEs.
inert even
Well – modern desalination plants work by using reverse osmosis (membranes/filtration) which is much more efficient for large volumes of reasonable fresh water than anything mentioned above.
Why not using the “old” method, Open air Salt pools? Since ancient times, people used to harvest salt fom the sea, using open air pools filled with tidal sea water. Close it and wait some time (weeks, months) and extract the salt. The evaporated water will rain somewhere else and fuel not only our needs but all of nature.
To make it a bit more efficient (and remember, this is a solution for places like Dubai or so, with massive amounts of land and economical resources.) take a place with predominant sea winds. Make the open air pools. Then make a mount downwind from the pools and plant adequate vegetation. With the right combination, these would trap the moisture and fuel a green ecosystem. Make it big enough (thus recreating the natural water cycle) and voilá.
I always feel nauseated when I see all the spending happening in Dubai to produce an artificial Paradise, when in fact they are producing another Urban Nightmare…In 100 years time there will be nothing but ruins and desert there…
The above simple process works but terribly inefficient. Salt water is not a great electrolyte for efficient electrolysis and especially bad at room temp and pressure.
The other issue is the constant replacement or cleaning of the electrodes since water has all sorts of unwanted minerals.
I wonder why fresh water is not managed or stored more efficiently. Why do we need to run the toilets with drinking quality clean water? Perhaps two pipes into the home – clean and drinkable? Also, it seems large amounts of rainwater water gets drained into the sea with poor water management policies. I’m thinking south florida and the everglades.
The original question remains. Given the inefficiencies of the chemical changes, how much electricity needs to be purchased to keep the process going and from that process how much water is produced?
2 related issues:
1. We are always drawn to the “high tech” solution when low tech makes so much more sense. Nuclear plants to generate electricity for instance over solar cells. Solar cells over concentrated solar etc.
2. I think mechanics illustrated had articles way back about cutting a trench from the Red Sea to some lower land area. All along the trench you placed hydro-electrical generators. Seems like with evaporation and what not it would take 50 years or so for the system to become uneconomic as the low area filled with water but I think one design said with proper design getting less electricity per year the system could go forever==not taking into account climate shift with global warming.
It’s a question of energy. We need a leap in energy production. Even fission or fusion plants are just heat for boilers to turn turbines. It’s a slight improvement from burning wood or fossil fuels. A source that negates the Inefficient heat, to mechanical to electrical conversion is needed. This is where basic research in particle physics will hopefully pay off.
#13 “Well – modern desalination plants work by using reverse osmosis (membranes/filtration)”
Apparently, the most modern ones use nuc power.
“In Japan, some ten desalination facilities linked to pressurised water reactors operating for electricity production have yielded 1000-3000 m³/day each of potable water,”
In my younger days I would do chemical experiments (graduated in chemistry eventually) and I tried to improve the production of hydrogen by adding salt NaCl to the water. Was surprised to seen green chlorine gas bubbling out. A lot of ideas end up as dead ends.
http://www.solaqua.com/
I use this at at work
I can’t follow the formula’s here, but it implies that with some heat capture Marc’s idea might work?
http://www.stardrivedevice.com/electrolysis.html
#18 Paddy-O. You’ve got it. Read my post #7 above. It’s much the same thing but on a much larger scale.
It kills me that the solution can be in place in 5-7 years but isn’t going to happen.
Just use a vapor compression distiller, powered by solar cells or windmill power. No coal, wood, nuclear power needed.
Another stumbling block is the cost of a fuel cell. They use platinum as a catalyst. This is a rare and expensive metal. There are many much cheaper ways to make fresh water from salt.
Although in many cases it would be even cheaper to be more careful with the fresh water we already have.
#24 “Just use a vapor compression distiller, powered by solar cells or windmill power. No coal, wood, nuclear power needed.”
Workable for low output needs.
Everyone has missed the real answer to “why wouldn’t it work”… COST. How much does it cost to deliver a river of water? Any good solution has to have a low production and maintenance cost
Thermal desalination can be MUCH more energy efficient and MUCH less costly than RO and electrolysis or electrodialysis. Even today’s good thermal technologies use less than 4kW-h/cu.m. plus some amount ( not very large)of WASTE ( low grade)heat.
In any case they are much more efficient(means cheaper) than the proposed one
The level of relative desalination is key to this process and its efficiency. If the water is a second stage desalination product, with consequently high concentrations of dissolved salts, the desalination benefits can be economic using electrolysis. Also if electrolysis is combined with thermal and kinetic dynamics the results can be very economical. Finally, if the energy used is waste heat, from some other process the economics can be very favorable. In our drought reduction via mechanical desalination plan, as viewed at gravitationalsystems commercial website, we employ a thermal separation vortex with electrolysis to generate a low quality desalinated product that when vectored into the upper atmosphere supplies both the water and salt nuclei necessary to generate rainfall.
If you use salt water (h20 with sodium chloride) you will produce chlorine gas at the positive terminal, not oxygen.
If you can’t afford the catalyst why not burn simply burn the hydrogen and oxygen, the end result steam would be at a high enough temperature to use an efficient brayton cycle, and since you’ve now got pure steam you can just cool it, by venting it straight into the local reservoir; there’s your fresh water!
Even if you don’t get too much energy back out of it, I wouldn’t be surprised if it was more efficient then reverse osmosis (not to mention replacing filters) and the pleasantly simple, yet horribly inefficient boiling and condensing water system, plus this has the added advantage of being able to produce salt, sodium and chlorine (dedpending on water and hydrolysis conditions) which can be processed and sold to recoup more of the cost.
Unlike R.O. the water would have zero contamination at all, and would be exceedingly pure.
You said 80% efficiency of reclamation. Make that 50%, unless you want to buy expensive equipment to heat the hydrogen and oxygen to a high temperature(which defeats the purpose anyways). Plug in the fact that it takes 1.25 V to seperate one mL of water, and you find that it take more than 600 joules to purify a liter, versus about 180 for regular ol’ distillation. When you use the heat of the steam to evaporate more water, it gets to about 90 joules.
So yeah, a nice idea in theory, but in practice, its too inefficient.